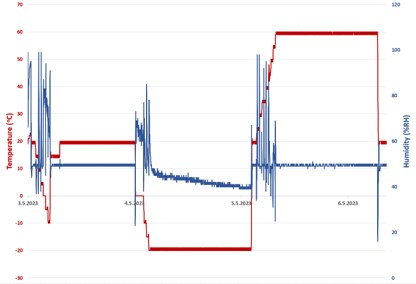
See Vac
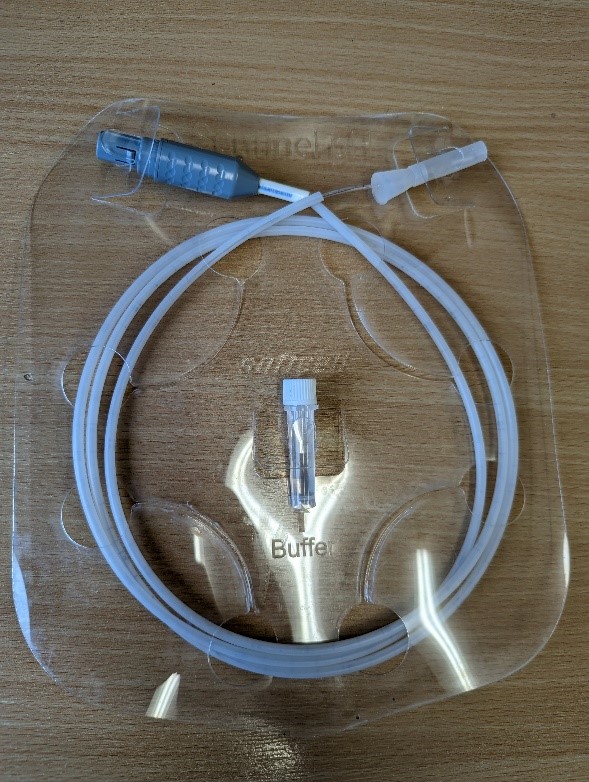
Injection moulding of dental device
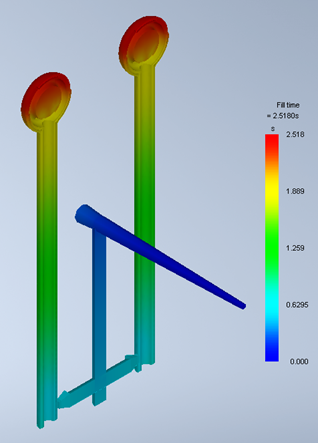
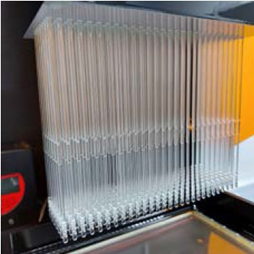
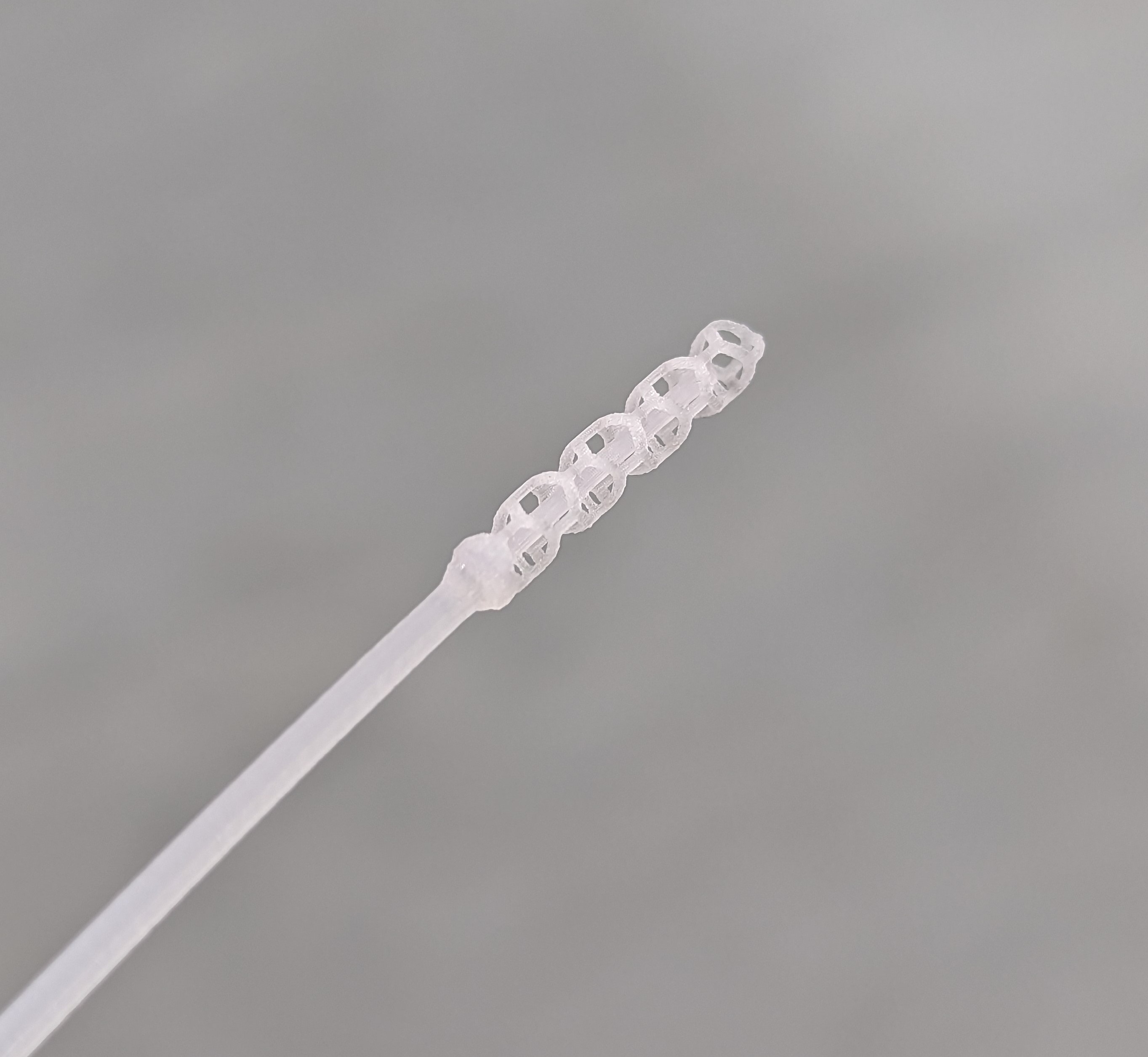
The purpose of the device in question is to facilitate capabilities of a suction tool and a mirror tool within a single dental device. The desired manufacturing method was injection moulding, which required mould tool design and mould flow simulation, followed by a small-batch production to identify well performing injection parameters.
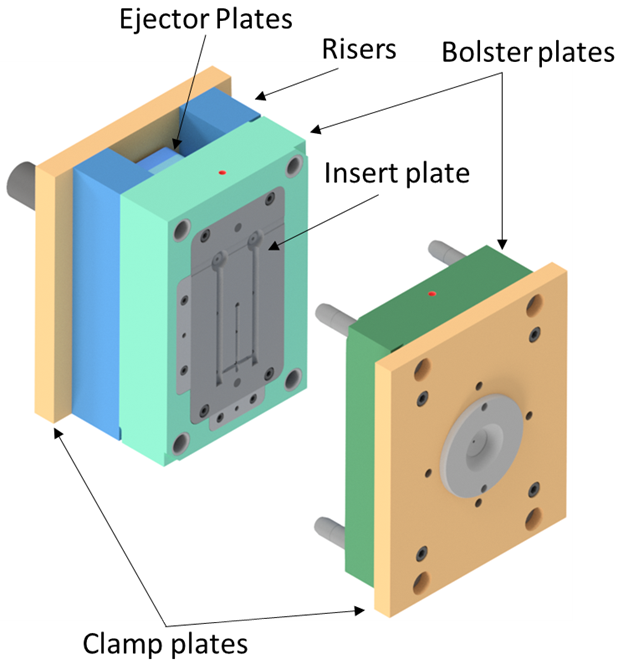
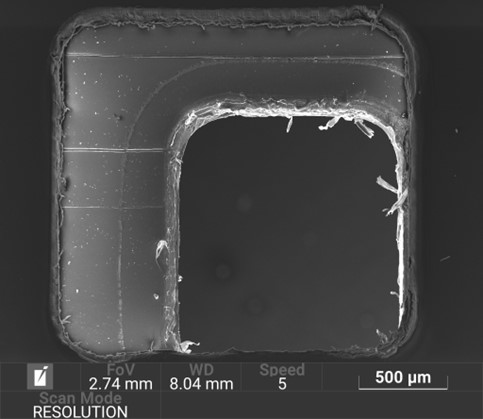
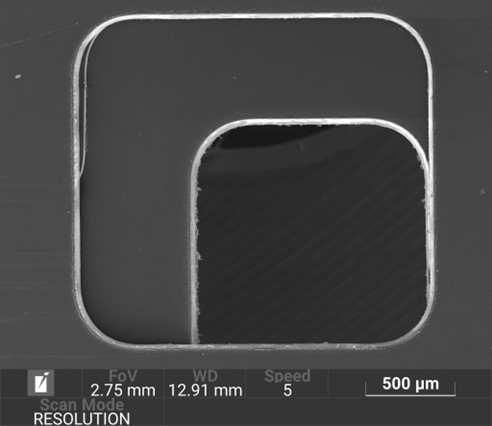
The mould tool is computationally modelled with manufacturability in mind. The insert plates can be easily removed and replaced within the bolster plates. This is a commonly employed workflow-streamlining approach found in many injection moulding facilities.
The mould tools are manufactured by a spark erosion process which incorporates the use of copper electrodes. The entire injection moulding pipeline is facilitated in-house: the electrodes are designed and CNC milled to the desired geometry, which are then used in the spark erosion process to manufacture the final mould plates.
"A huge "Thank You" for helping me last year with all the hard work you have put into doing the tooling for See Vac … I couldn't have got to this stage without the help of MDMC"
- Louise Kennedy (Founder)