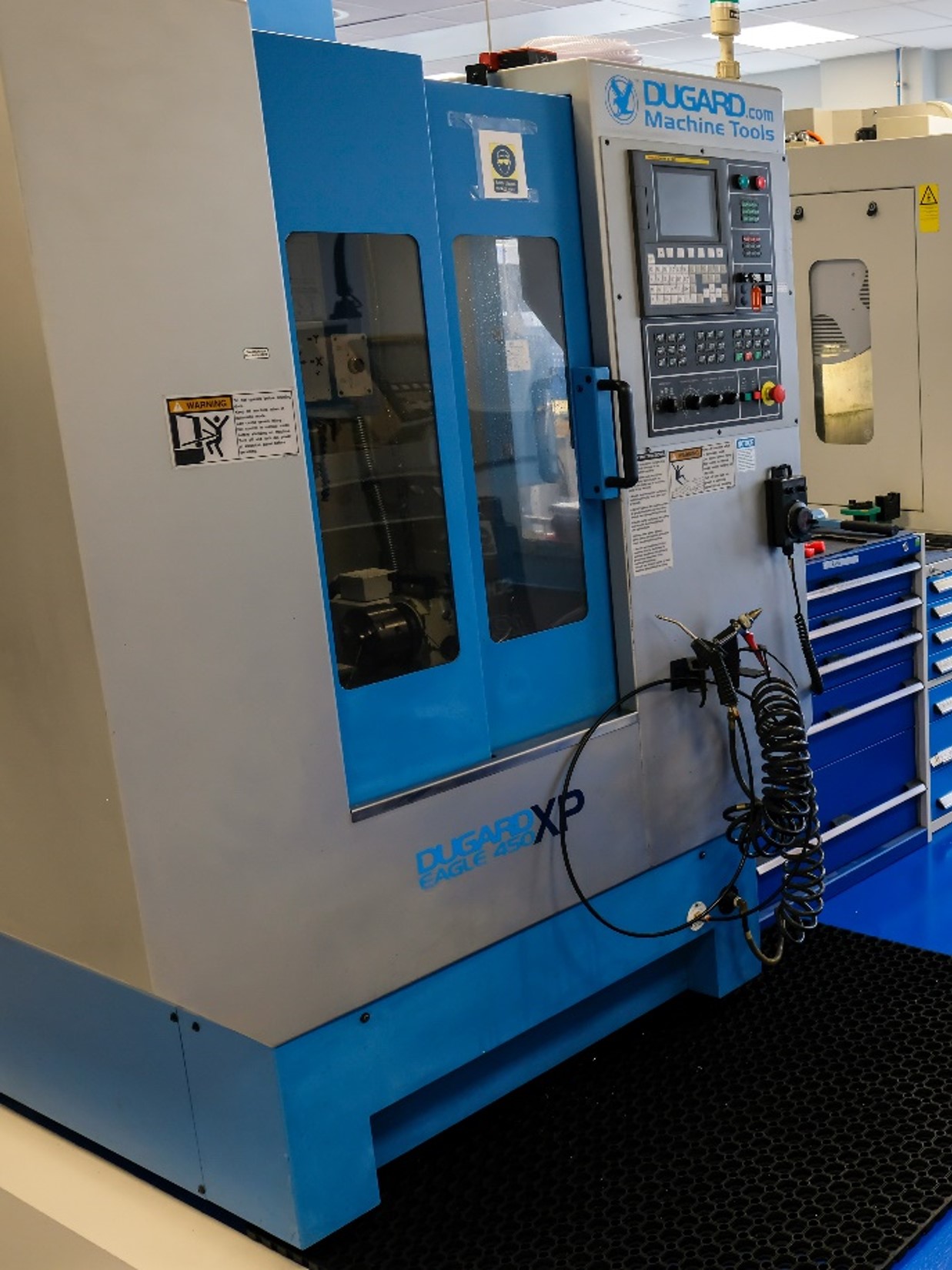
We provide medical device developers and manufacturers with the advice, technical expertise, and facilities essential for companies that are seeking to translate medical device concepts to commercial products. Our mission is to provide expert advice on manufacturing, engineering, regulatory issues and funding, coupled with technically supported access to manufacturing facilities. We aim to assist Scottish companies in the translation of medical device concepts through to small batch commercial prototypes. Services provided by the MDMC are free of charge to Scottish Companies.


We are a highly experienced inter-disciplinary team composed of engineers, scientists, and clinicians across the 5 universities.

As Director, I am responsible for leadership and strategic direction of MDMC across the 5 university partners, and provide particular expertise on laser-based manufacturing processes.

As PI and manager of the MDMC, I supervise the HWU Technology Specialists and am responsible for the day-to-day running of the Centre. I also look at the sustainability of the MDMC from 2026 onwards. I specialise in systems integration and novel manufacturing processes.

As a Business Development Executive, I develop relationships between the MDMC and medical device manufacturing Companies. I provide initial support for prospective clients and identify new opportunities for the MDMC to enhance relationships with industry.

As a Technology Specialist, I take on technical responsibility for the success of client applications. I am the technical expert on laser-based manufacturing, liaising between SMEs and MDMC and providing solutions to ongoing and strategic enquiries.

As a Technology Specialist, I partake in client interactions by providing technical expertise on prototyping, additive and subtractive manufacturing, imaging, and analysis, promoting solutions that successfully meet client needs.

I am a technology specialist experienced in medical device research and development. Using the facilities at the MDMC, my role is to provide companies expertise and support as they develop and manufacture their medical devices.

I am a Technology Specialist, providing companies with expertise in the development of their medical devices and products. My primary goal is to find solutions which successfully accelerate the projects of our clients.

As a technology specialist in medical device research and development, I focus on assisting companies in advancing their products. My role is to offer technical expertise and support to accelerate client projects and ensure effective solutions

As Project Manager I am responsible for the day-to-day management of MDMC across the 5 universities, ensuring deadlines and deliverables are achieved across the consortium while proactively managing close working links with key internal and external stakeholders.

As BDE, I improve collaboration between the MDMC and Scottish MedTech manufacturers, SMEs and larger organisations to provide our innovative services. I provide initial and follow-up support for clients and identify new opportunities for the MDMC for future growth.

I am a registered nurse with med-tech, clinical trials, and preclinical testing experience, including developing Thiel embalmed cadavers for preclinical research and training. I am driving the Tayside Innovation MedTech Ecosystem (TIME), introducing new healthcare technologies.

As a Quality Assurance Coordinator, I design and implement a comprehensive quality management system for our Dundee facilities. I drive compliance with regulatory and organizational standards and optimize operational efficiency through cross-functional collaboration.

As a research technician with 12 years experience in surgical simulation, I provide the expertise required to deliver cadaveric simulation models. With an emphasis on imaging, I deliver projects enabling pre-clinical testing of medical devices.

I am a Technology specialist providing engineering assistance to medical device companies at various stages of device development. My expertise include prototyping, materials testing as well as various simulation and modelling strategies.

I am a technology specialist with a background in design engineering & research within the medical device industry. I bring my expertise to aid client projects in the prototyping, manufacture, and testing of novel medical devices with a focus on precision metalwork.

I am a technology specialist with a background in the development and manufacture of novel medical device prototypes. Working within the medical and oil and gas industries I have more than ten years’ experience in precision engineering and manufacturing.

I am the project administrator for MDMC within TIME, based in Dundee. I am the principal point of contact and provide the appropriate support to log, file and report information and ensure follow-up actions are completed after meetings.

My role in MDMC is to provide a link with opportunities and equipment to the highly active medical devices community at the University of Glasgow. My own manufacturing specialism is in mesoscale fabrication, with microultrasound devices as a specific target.

I am a medical statistician specialising in clinical trials, with previous experience as a consultant statistician in the life sciences industry. I have expertise in the design and conduct of medical device trials, both in academia and industry, including regulatory knowledge.

As a Project Financial Manager, I look after the financial aspects of the MDMC including liaising with project partners on budgets, actuals, ensuring compliance and accuracy of claims, etc.

I lead the research group on Sensors and Instrumentation at the School of Engineering, Robert Gordon University, providing particular expertise on manufacturing of biosensors and related instrumentation.

I have a long career in nurse education at the University of Edinburgh becoming Professor of Student Learning (Nurse Education) in 2012 and holding key responsibilities in the undergraduate and post graduate nursing programmes.

Professor Lis Neubeck is a cardiac nurse and Head of the Centre for Cardiovascular Health at Edinburgh Napier University. Her research includes management of atrial fibrillation and digital health.

I am the Clinical Director at the Large Animal Research and Imaging Facility at the Roslin Institute and in this role organize and manage any safety and efficacy studies required: i) in the development of medical devices and ii) to meet any subsequent regulatory requirements.

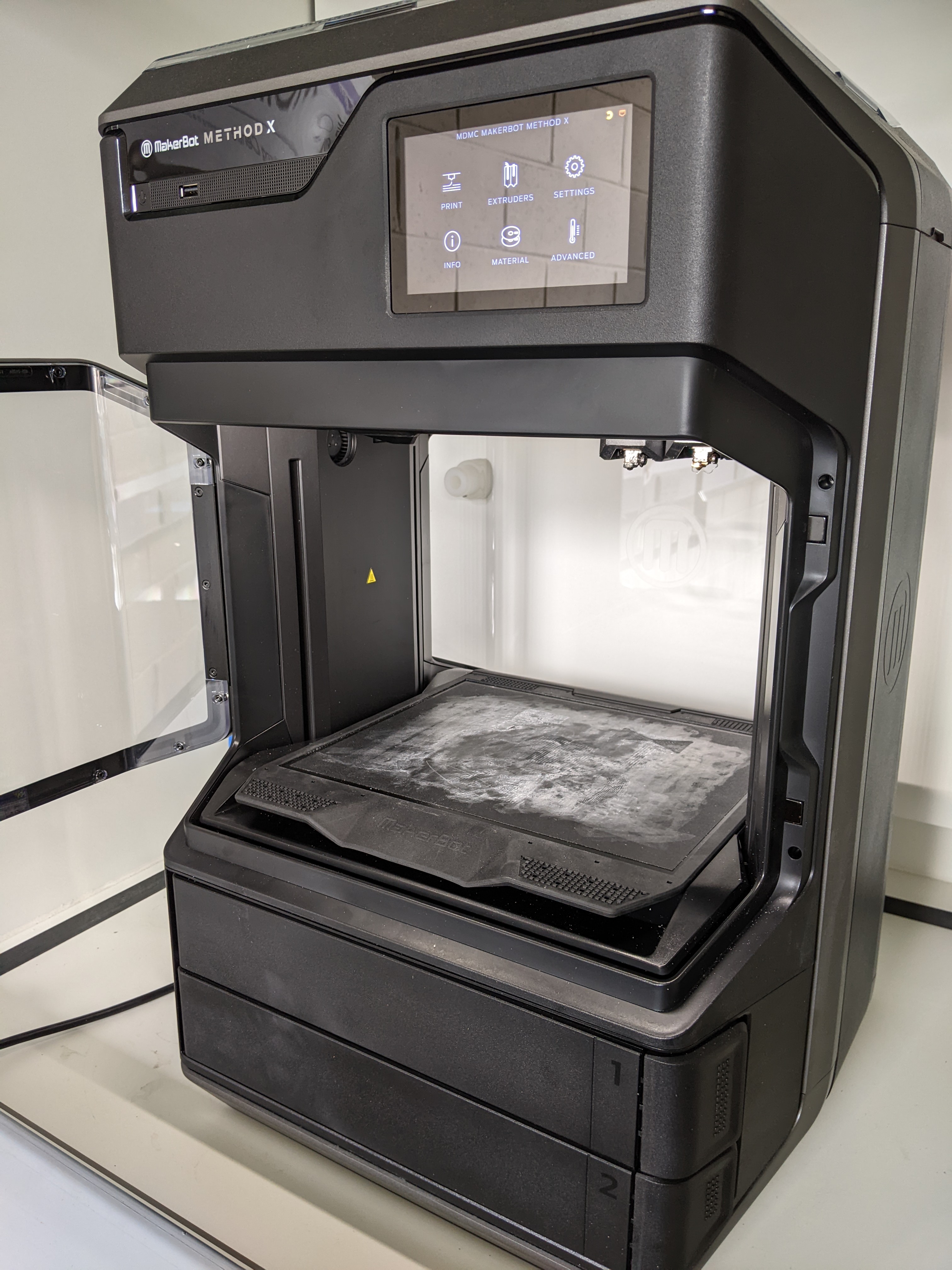
















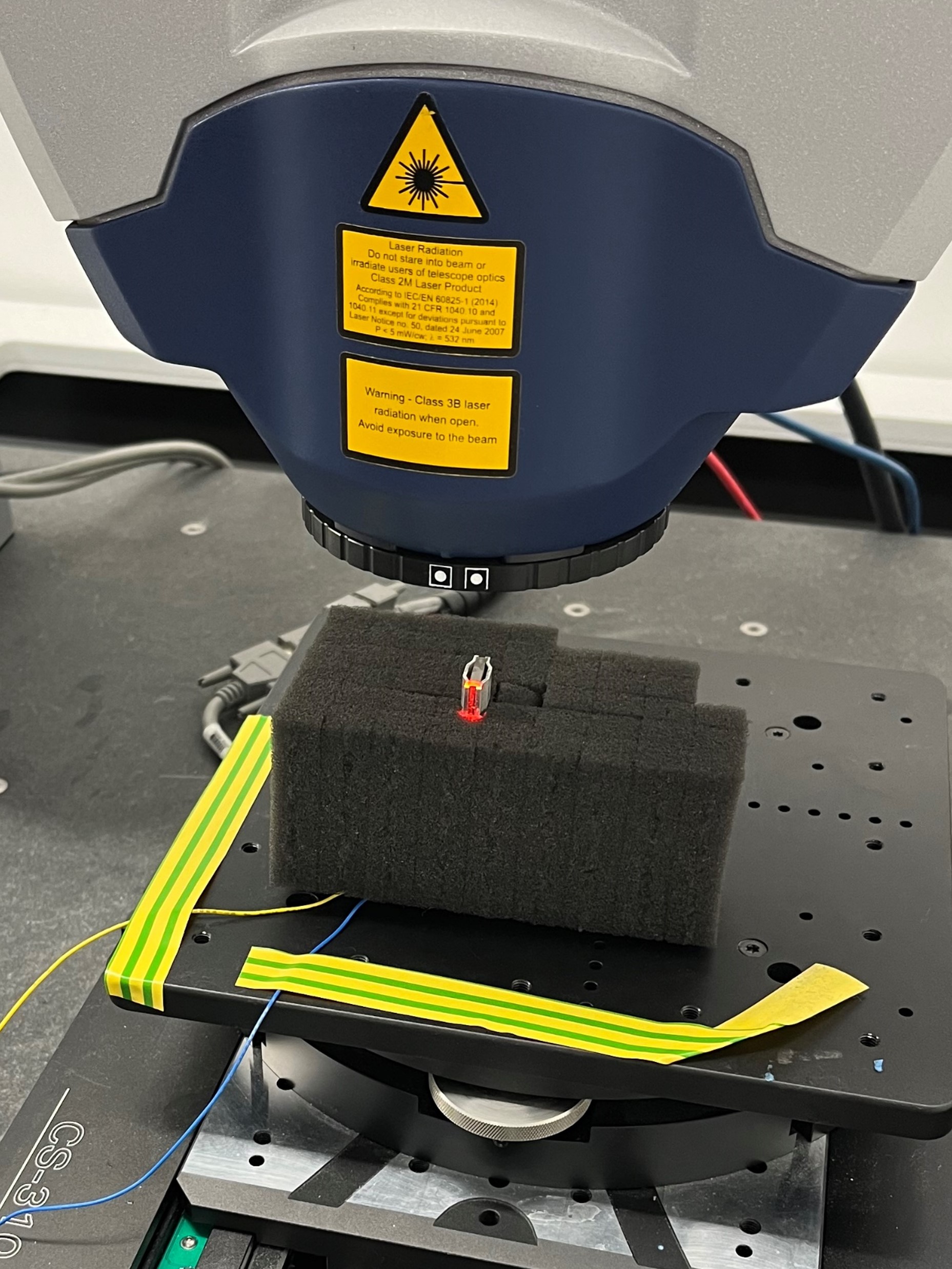









If you would like to get in touch or have any enquires regarding access to the MDMC services, please complete the MDMC Enquiry Form linked below and we will get back to you as soon as possible!